ICU-grade LibreMask
What is LibreMask?
LibreMask is intended to be an ICU grade mask that uses breathing filters, HMEs and HMEFs filters in order to achieve a filtering efficiency superior to N95 or FFP2 masks.

LibreMask has been developed by a group of Spanish researchers and citizens, with the help of a number of companies joint under the Coronavirus Makers [2] movement.
- First prototype hot press molding provided by Eyser Hydraulica [3].
- Second prototype and hot press molding provided by Coeca [4] and Tecnasa [5].
- Third prototype and hot press molding provided by Coeca and Tecnasa, and funded by Plastic Oceans [6].
Libremask design consists of a mask made by hot press molding or mold injection with silicone or santoprene, removing the caveats of 3D printed materials, and a standard breathing filter, available in hospitals. These filters offer filtering efficiencies greater than N95/FFP2 masks, shielding the most penetrating aerosol size - 300nm - in comparison to surgical masks that offer shielding to micron-sized aerosol. These filters even outperform conventional N95/FFP2 masks as reported by the manufacturers [1], and by non-standard aerosol filtration tests described below.
It is based on the “Reusable Elastomeric Respirator“ developed by Boston Children’s Hospital and Harvard Medical School. [7]
You can copy, modify and reuse all this material in part or totality as long as the resulting product is maintained with the same open hardware license. The mask is not certified, use it at your own responsibility.
Aerosol non-standard tests
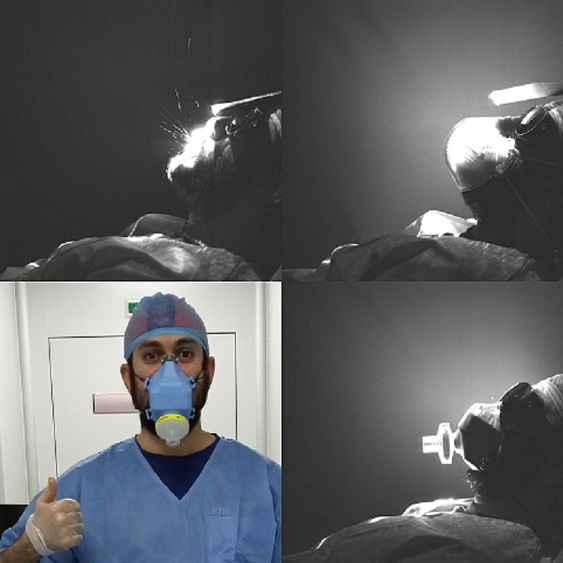
Following the recommendations from the Spanish National Research Council (CSIC) [8], in the pandemic context, we replicated the experimental setup thanks to the Interuniversity Institute for Earth System Research in Andalusia [9], at the University of Granada, to measure filtering capabilities against the most penetrating aerosol size, 300 nm. Nevertheless, these are not the EU standards that are addressed in the following section.
Generation of NaCl aerosol and size selection with an electrostatic column, at 300 nm-size. Aerosols are counted before and after the filter with two particle counters at the same time, at 1.5 liters per minute.
| Component under test | Filtering efficiency |
|---|---|
| FFP3 mask | (99.999985±0.000004) % |
| Breathing filter | (99.99909±0.00004) % |
| Breathing filter used for 5h30m with 30m of intense physical activity | (99.666±0.006) % |
| Surgical mask | (75.9±0.8) % |
European standard tests in AITEX
Tests performed in the European public certification entity AITEX (in Spanish: Asociación de Investigación de la Industria Textil). We prepared the masks with head harnesses, and we sent 10 units and 10 filters to AITEX to perform tests of i) resistance to breathing and ii) paraffin oil aerosol filtering test. We decided to change to Flo-Guard filters from Intersurgical [11] which specifically present the lowest resistance to breathing while keeping the filtering efficiency. They are simply larger in diameter than the Inter-Guard filters.
Note on EU standards: The EU standard followed by these filters is the "EN ISO 23328-1:2008 Breathing system filters for anaesthetic and respiratory use. Salt test method to assess filtration performance". Thus, only salt (NaCl) aerosol is used to test their filtering efficiency against microorganisms, and for this reason Intersurgical can state that these filters are anti-virus. When attempting to use the same filter as a PPE, a different EU standard applies, the "UNE-EN 1827:1999+A1:2010 Respiratory protective devices - Half masks without inhalation valves and with separable filters to protect against gases or gases and particles or particles only - Requirements, testing, marking". This EU standard increases the requirements of resistance to breathing and filtering efficiency. In particular, the masks need to be tested with both paraffin oil aerosol and NaCl aerosol.
Paraffin oil aerosol is not representative of pathogenic virus size (>1 μm) nor composition. By size, it should be easier to stop the paraffin oil aerosol by inertia. Nevertheless, paraffin oil tends to decrease the performance of electrostatic filters as it is the case of the filters we propose. A recent European PPE regulation made during the pandemics, PPE-R/02.075 version 2 [12], removes this test. Under these regulations manufacturers need to state in the packaging that the mask is a “Filtering half mask to protect against COVID-19”. Unfortunately, such regulation is only available for one-use disposable masks, and LibreMask is reusable and filters can be replaced, and therefore it does not meet the requirements for this special regulation. For comparison, N95 and N99 NIOSH American standard does not test the paraffin oil aerosol. But we had to, and we did so. And the results are as follow:
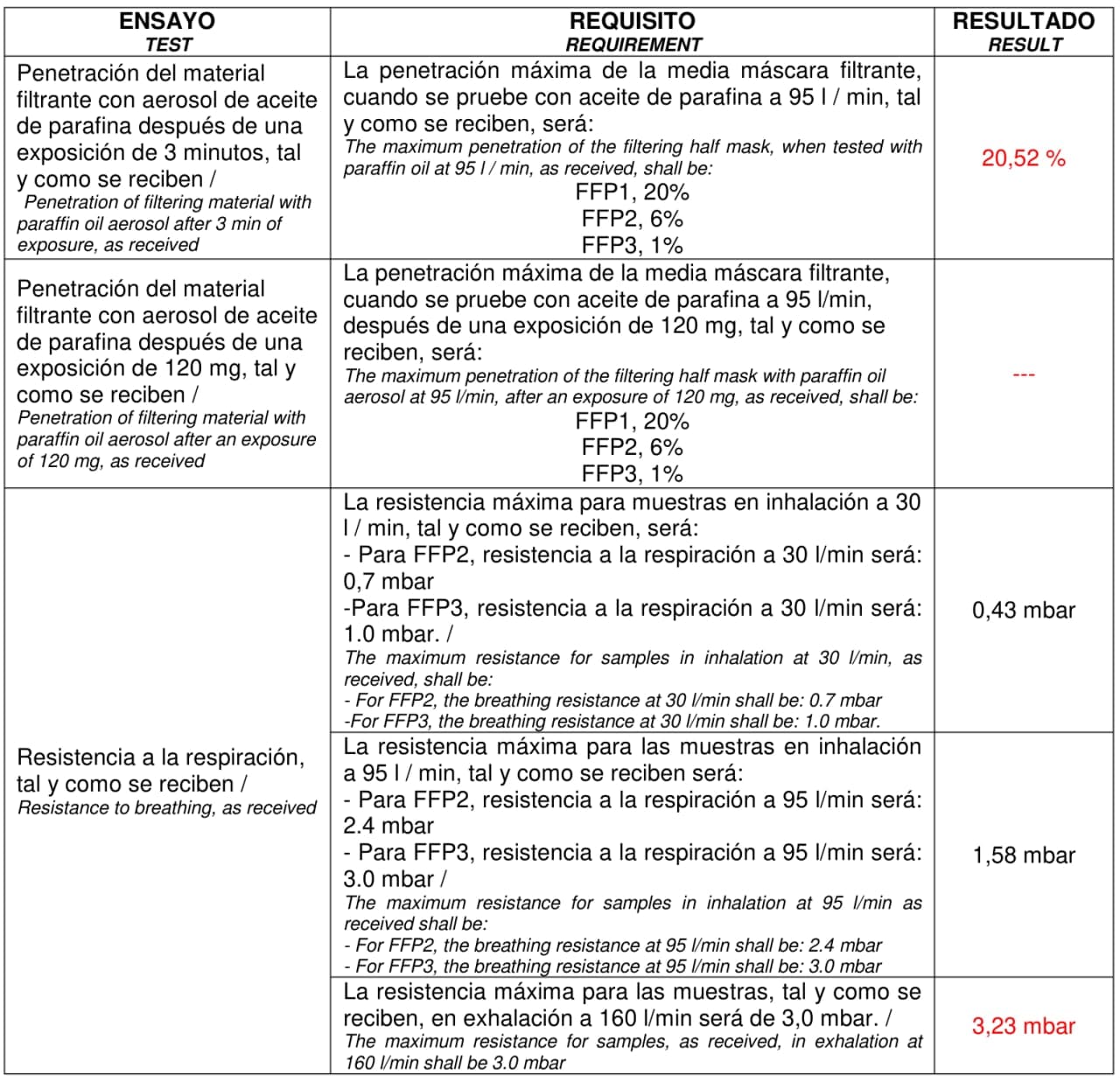
As mentioned above, the results of filtering efficiency against paraffin aerosol were bad as expected. This implies this combination of mask + filter cannot follow such EU standards. It could in principle follow the special “Filtering half mask to protect against COVID-19” but it would defy one of the basics of this solution: to be reusable and to use the filters available in hospitals. It might be still a valid N95 or N99 mask under NIOSH American standards, and we encourage their testing according to the very positive results of resistance to breathing of our tests, and knowing that they already are tested against NaCl aerosol following the European standard EN ISO 23328-1:2008 that mostly adapts the NIOSH NaCl aerosol test.
As a summary, due to the regulations not adapted to the present situation, in Europe we will not be able to certify this mask, not even following the special regulation due to COVID-19 as this regulation is not available for reusable masks. We therefore encourage any country following the NIOSH or any other similar regulation to test them with all the information that we gathered so far.
How to replicate it
LibreMask is libre open source hardware. You are allowed to copy, modify and study the manufacturing files. Moreover, you are allowed to use the manufacturing files to create your own LibreMask
While LibreMask has been tested as described above, it is not fully certified and we cannot warrant that it will protect you against coronavirus, or other airborne diseases.
Hereby, use it at your own reponsibility. Any person or company taking over this project should test them to fully certify its filtering efficiency before donating or selling them.
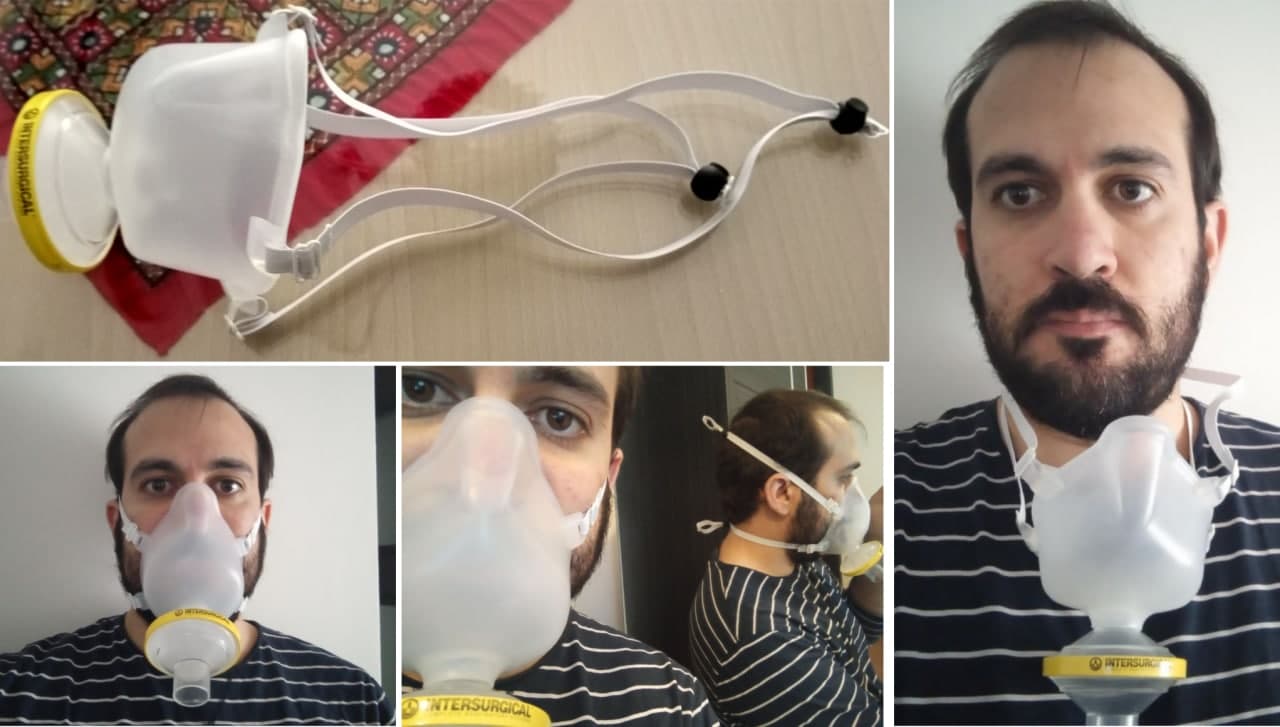
Libremask is composed of:
- A silicone/santoprene hot pressed/injection molded mask
- A breathing filter
- Two elastic straps
Manufacturing files
The following files are available to manufacture the mask :
- Step and stl file of current version of the mask.[14,15]
- Soft tooling step file for 3D printing a mold. [16]
Libremask is hot press/injection molded, avoiding the concerns on the porosity and mechanical properties of conventional 3D printed masks.
Nevertheless, fabricating the mold by 3D printing and injecting silicone also avoids 3D printing problems thanks to the wettability between the liquid silicone and the 3D printed mold.
Breathing filter
LibreMask uses a standard breathing filter. The result of our non-standard tests are based on Inter-Guard breathing filters [1], from Intersurgical. The tests in AITEX (UE public certification entity) were performed with Flo-Guard breathing filters [11], from the same manufacturer. Any other certified breathing filter will perform the same as they are standardized both in dimensions of the connection and filtering capabilities. The decision to use these filters came as the availability of this product in Spain was not compromised during the first pandemic peak. Product datasheet claims a 99.99% filtering efficiency for 300 nm (NaCl) aerosol, which is consistent with the result of the trials performed in Universty of Granada.
Elastic strips
You can source locally the elastic strips. This is intended to be a consumable that can be replaced as frequently as needed. They should firmly press the mask against the face of the wearer, without leaving any space that could result in air-leakage.
Operating instructions
Assuming the mask is sterilized as described below, a breathing filter needs to be press-fit installed on the mask, and the strips need to be checked and replaced by the strip of your convenience if they show signs of wear. Ensure it is air-tight on your face by blocking the filter with your hand and breathing gently, the mask should resist both gentle inhalations and exhalations.
Maintenance instructions
Given the results of filtering capability over time of the filters reported in the table above, we envisage that one breathing filter is used per working shift. After the working shift, the filter should be trashed and the material of the mask enables its sterilization by any conventional method, ranging from autoclave to immersion in diluted bleach, or wiped with alcohol.
Prototypes and development
Several trials have been performed prior to achieving the current LibreMask prototype. As described above, the final result is a hot press molded food grade silicone mask. It is equipped with a breathing filter, providing filtering efficiencies above N95/FFP2 masks.
During development 3D printing was used for prototyping. There are concerns about using 3D printing for the final mask as it might be porous and not reliable from one mask to the next. For this reason the final mask needs to be hot press or injection molded with santoprene or silicone, or any other food grade suitable elastomer.
In each stage, the masks were distributed to health staff in hospitals accross Spain to get direct feedback, and to improve it with their suggestions the mask, up to the current prototype.

Acknowledgements
The results of this project are due to a huge citizen effort and it would be impossible to acknowledge every single person that contributed. Dr Ramsés Marrero, Dr Vicente Pastrana, Dr Julia Herrera, David, Da, Pedro, Carmen, and many other health care workers that helped us to realise what they need in a very hostile environment as it is an ICU or ER in a hospital. Rubén that helped us with the technicalities of filters. Javier Montero and Armando Romanos and all the staff in IAVANTE (Junta de Andalucía) which offered us their clean room and equipment to test the masks, and sent them to hospitals to be tested. Prof. Lucas Alados and all the team in the University of Granada that enabled us to compare the efficiency of the breathing filters and FFP3 (N99) masks against 300 nm aerosols. All the volunteers in coronavirusmakers.org that managed to move 1000 km the masks in the middle of a holiday with the most severe mobility restrictions during the pandemics. And the football team that sponsored such expensive action, the Mérida Asociación Deportiva. The rail-workers from RENFE that carried the masks in the trains to distribute them across Spain. The policemen that distributed the masks to hospitals to be tested during the most severe mobility restrictions. Rosa from frenalacurva.net who provided the funding to test the masks in AITEX and made possible to have the results before 2020 ended. Antonio Novo from clusters.es, and all the colleagues from coronavirusmakers.org, that helped to give visibility to this project. And we cannot stress more the role that people like Ioseba Monje and his team, Miguel Carrasco, or Mike Bilodeau played, by financing and producing the masks, but overall, by making sure they reached the hospitals to be tested by the people that needed them the most. This last sentence will serve to the hundreds of persons that helped in one or another way, to make this project what it is, something to be proud of.
Team





Antonio Ordúñez
Design and testing of prototypes.

Iván Blasco
Manufacturing, conventional liquid silicone casting.




Santiago Andreu Carmona
Documentation.



Dr. Julia Herrera
Documentation, testing and quality control.


David Gil Sánchez, Nurse
Quality control.
Sponsors
Developer communities

Contact
For further information contact Miguel Ángel Fernández
References
- Intersurgical. Inter-Guard filter information [Link]
- Coronavirus Makers Movement webpage[Link]
- Eyser Hidraulica webpage[Link]
- Coeca webpage [Link]
- Tecnasa webpage[Link]
- Plastic Oceans webpage[Link]
- Boston Children’s Hospital. COVID-19: Our response to the N95 shortage[Link]
- Minguillón et al. Emisión y exposición a SARS-CoV-2 y opciones de filtración, Instituto de Diagnóstico Ambiental y Estudios del Agua (IDAEA), CSIC, v3, 15.04.2020 [Link]
- Andalusian Inter-University Institute for Earth System Research (IISTA-CEAMA) webpage [Link]
- Instituto Interuniversitario de Investigación del Sistema Tierra en Andalucía, Universidad de Granada. Eficiencia de filtrado de distintos materiales ante partículas de 300 nm. Exposición a aerosoles en SARS-COV-2. [Link]
- Intersurgical. Inter-Flow filter information [Link]
- PPE-R/02.075 version 2[Link]
- Report from AITEX European public certification entity [Link]
- LibreMask final step file [Link]
- LibreMask final stl file [Link]
- LibreMask tooling step file [Link]



